Mono and Multiple Sclerosis: Mapping the Red Flags of Disease With Network Models

Sometimes, good science takes time. To understand the true impacts of a disease or treatment, longitudinal clinical studies often require gold-standard lab data and methods that are both high effort and high cost. When patients’ health and lives are at stake, there is little room for error. Concurrently, new software tools and the comprehensive datasets that power those tools are creating opportunities for accurate, in-depth clinical insights to be delivered in a fraction of the time and cost, compared with legacy tools. Complex diseases, such as multiple sclerosis (MS), need a diversification of research approaches, as answers to some of the most basic questions are still yet to be found. Identifying where and when new technologies can complement, support, or replace other methods is key as we grow into the current era of data and computation.
Mapping a New Path
In January of this year, Science published a study validating the hypothesized link between Epstein-Barr virus (EBV) to multiple sclerosis. With data from the Department of Defense Serum Repository (DoDSR) collected over two decades, the study found that patients with EBV (a leading cause of mononucleosis) were 32 times more likely to develop MS than patients who had never had EBV. The study was built on the DoDSR’s database of ~10 million patients, of which 955 had multiple sclerosis.
A sample that large is considered a gold standard in clinical research. But additional approaches are needed when such standards are costly and can take decades to compile. Significant insights from a larger and even more representative patient population can be found much more quickly and easily by using patient journey data and advanced analytics. To demonstrate the speed, accuracy, and nuance that Komodo’s software platform can derive from real-world data, we replicated a version of this Science study using our Healthcare Map™ and software platform, tapping into 330 million unique patients, of whom 1.4 million had a multiple sclerosis diagnosis code.
Using our technology platform accessed via Prism, we created a patient journey analysis of a subset of MS patients with an earlier mono diagnosis (used as a proxy for EBV).
Our analysis uncovered some of the comorbidities that appeared frequently within six months of an MS diagnosis for patients with both a mono and subsequent MS diagnosis:
- Essential (primary) hypertension (16%)
- Anxiety disorder, unspecified (18%)
- Hyperlipidemia (12%)
- Gastro-esophageal reflux disease without esophagitis (12%)
While many of these comorbidities appear at similar rates in the general population, there were three of note:
- Vitamin D deficiency (16%): Previously published literature suggests there may be a connection between low vitamin D amounts and autoimmune diseases.
- Hypothyroidism (12%): Also an autoimmune condition, hypothyroidism could be linked to mono in a way that is similar to how MS is related. Instead of autoantibodies attacking the nerve cells, as seen in MS, in hypothyroidism they attack thyroid cells.
- Other fatigue (23%): Fatigue is common in MS patients, and considered a hallmark symptom of the disease.
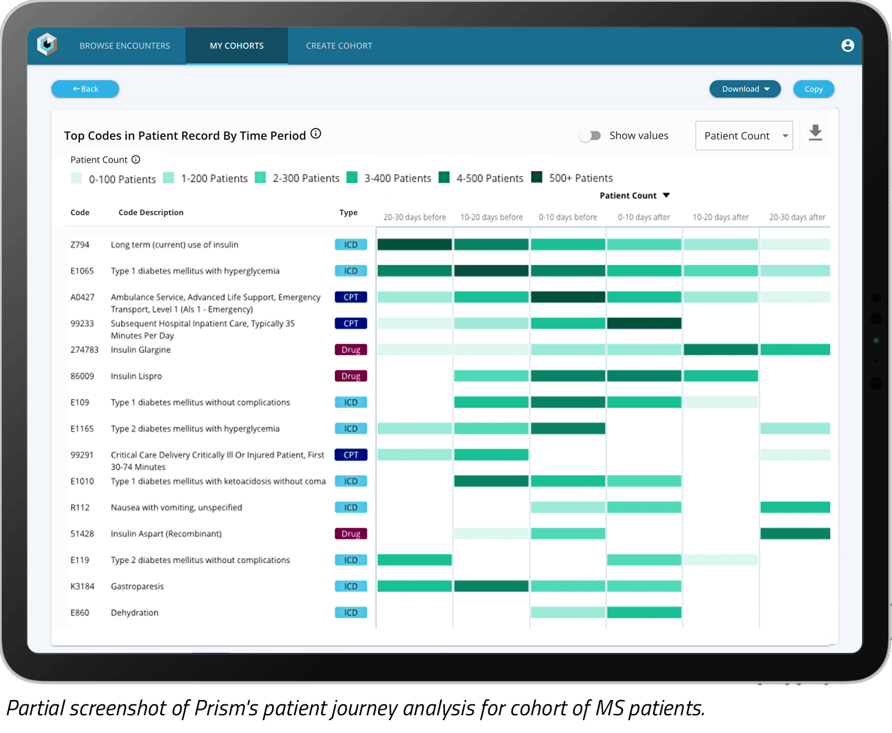
When treating patients with a previous mono diagnosis, the presence of indicated comorbidities may suggest the patient should be prescreened or evaluated for MS.
Understanding potential disease indicators or “red flags” in a patient’s journey to diagnosis can offer valuable insights into opportunities for early intervention, a faster time to diagnosis, and, ultimately, improved outcomes. This type of diagnosis journey mapping can also be useful in tracking the progression of a viral illness (such as mono or cytomegaloviral), offering us a better understanding of long-term impacts and outcomes.
Moving from Insights to Action
Novel patient journey approaches like the one used in this analysis and others provide the analytical scaffolding to solve a diversity of use cases — from evidence-generation strategy in clinical development, to market access strategy in the commercial space. Equipped with real-world evidence, teams can better:
- Optimize clinical trial design and eventual commercialization by modeling the predicted impacts of inclusion/exclusion criteria, and of real-world comorbidities and treatment pathways.
- Detect and correct for biases by overlaying demographic data such as race and ethnicity across a unique network to segment and flag any disparities in patient journeys to ensure all patients have the opportunity to access the novel treatments they need, faster.
- Identify and educate providers about key moments of interventions to help target and deliver therapies to the right patients, at the right time.
Datasets like the DoD serum repository have been the cornerstone of research initiatives, especially with diseases that have long, intricate, and nuanced diagnosis pathways. And while these sources include detailed laboratory data, it took nearly 20 years to collect enough information to generate the insights published Science. In chronic, debilitating disease states like MS, patients don’t have two decades to wait. Good science can take time, but shortening those timelines where and when we can will have significant real-world impact.
Where We Go Next
The advent of longitudinal RWE data and its inclusion in HEOR, clinical, and medical research offers a way to circumvent the challenges that come from legacy datasets. With near-real-time patient-level data, studies could be envisioned, replicated, and validated with an unprecedented level of speed and scale. Our analysis encompassed three times the number of patient lives and was conducted in a fraction of the time — only minutes of work from ideation to insight generation. While our report doesn’t answer every question, we were able to validate current thinking and generate new insights about the patient journey which could serve as evidence and directional guidance for future hypotheses. New research opportunities are also possible through linking additional de-identified datasets such as social determinants of health data, payer data, biomedical data, or clinical trial data.
We are only scratching the surface of what we may be able to uncover when we apply analytical approaches to complex, poorly understood disease states such as MS. The questions yet unasked will provide further insight into patient journeys that would be otherwise hidden, ultimately helping us to reduce the burden of disease for all.
To read more work based on Komodo Health’s analytical approaches, check out, “Applying Data Science Expertise to Komodo’s Platform To Answer Novel Healthcare Questions.”
To see more articles like this, follow Komodo Health on Twitter, LinkedIn, or YouTube, and visit Insights on our website.





