IMPROVING THE ODDS OF R&D SUCCESS
Smarter Clinical Development Strategies Start Here
What if we could take just a fraction of the uncertainty out of the R&D equation? What would the world look like if clinical trial enrollment rates grew by just 10%, or if even 20% of new compounds won FDA approval?
Improve the odds of R&D program success with Komodo Health’s 325,000,000 complete, real-world patient journeys and decades of clinical expertise. Place patients at the center of your clinical trial design, pinpoint precise population cohorts, rapidly identify the most productive trial sites, and reduce your total study time.
Take the unknowns out of your clinical development strategy.
STRESS TEST STUDY DESIGN
The smallest detail in a research protocol or endpoint selection can make or break the viability of a trial, and the success of a study often hinges on the ability to predict the barriers to trial participation at each study site. Komodo’s clinical development solutions make it possible to simulate protocol design, pressure test inclusion/exclusion criteria, and assess the geographic spread of eligible patients before initiating trial enrollment, reducing study times by over 30%.
ACCELERATE PATIENT RECRUITMENT
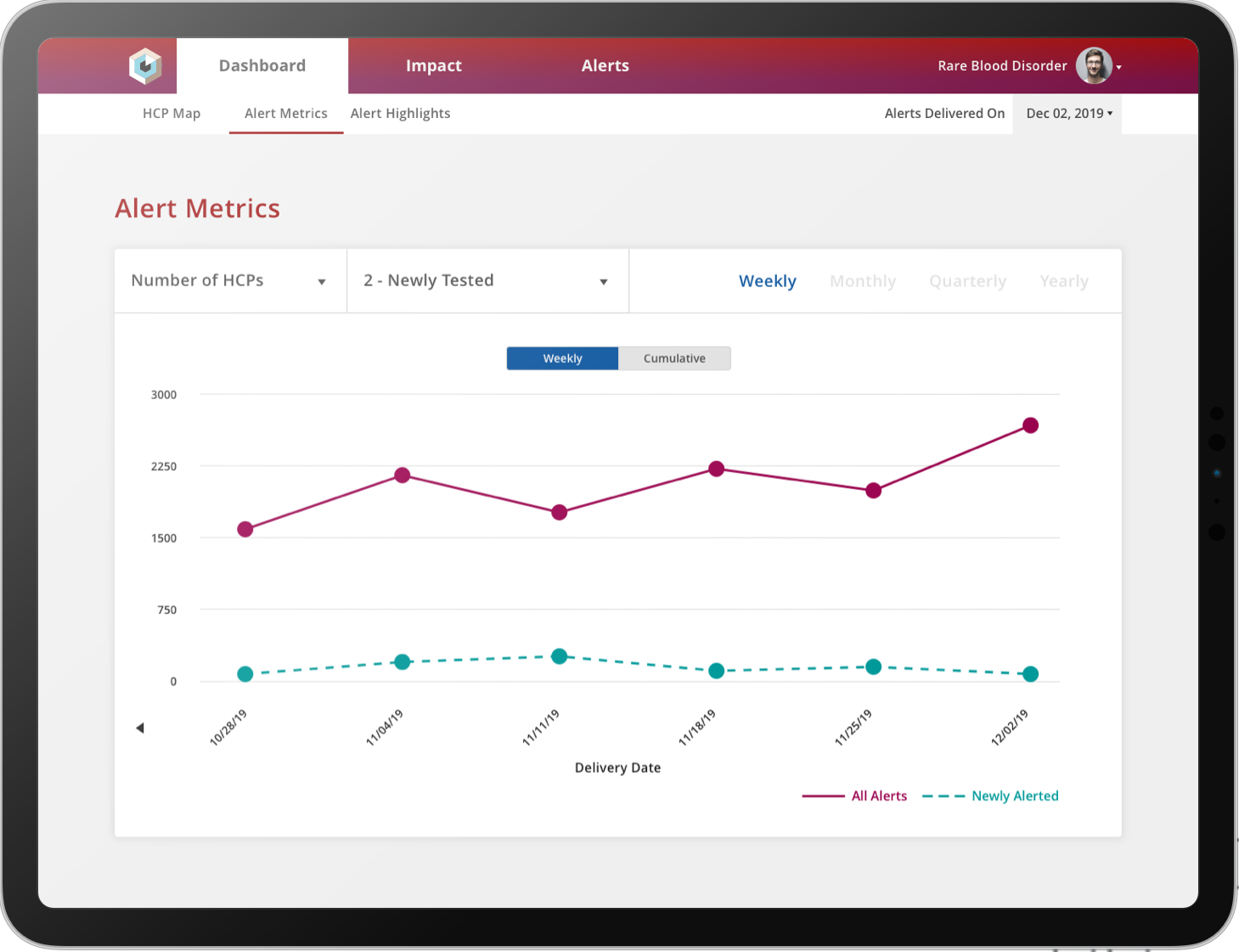
The ability to find screening-eligible patients at moments that matter can mean the difference between meeting enrollment goals and failure to launch. By delivering granular, near real-time, zip code – level alerts of real-world patient interactions with the healthcare system, Komodo has helped life sciences clients double the number of patients screened for trials, expediting enrollment timelines.
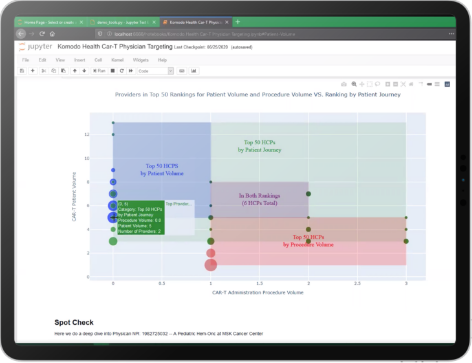
OPTIMIZE SITE SELECTION
A successful site versus the next dud depends on the ability to accurately predict performance. By seamlessly connecting clinical trial data with patient-level insight, our solutions have empowered R&D teams to understand site enrollment propensity, model and forecast their performance, and map centers of influence in a matter of minutes — saving upward of $150k in operational efficiency per site.
Enhance RWE Generation with the Most Precise View of Your Patient Cohorts
Drive Trial Diversity and Inclusion
Find underserved populations in Komodo’s Healthcare Map™, the industry’s largest and most complete database of de-identified, real-world patient journeys with census-level representation down to the zip code level anywhere in the country.Turbocharge Recruitment Speed
Get timely patient-level alerts, before treatment decisions are made, to enable differentiated, care-based messaging on trials, disease state and mechanism of action (MOA) with the HCPs who are actively treating screening-eligible patients.Design Higher Quality Studies
Understand patient journeys in granular detail with claims data that represent the diversity of the US population and capture 2-3 times more clinical encounters per patient than legacy data aggregators.Power RWE-Enabled Clinical Trials
Connect PROs, genomic, EHR and other patient-level datasets to the Healthcare Map™ in order to analyze trial data with deeper specificity, understand patient outliers and monitor outcomes even after study completion.Whether targeting a large volume of diverse patients for a prevalent chronic condition or exploring breakthroughs for complex rare diseases, it’s crucial to understand where patients are progressing through the care system in order to improve efficiency in clinical trials.
Komodo’s technology and real-time data helps thread the needle on everything from clinical sites and physician engagement to patients with relevant diagnoses.