Veozah's Early Journey: Analyzing Uptake of the New Drug for Treatment of Hot Flashes

In Komodo’s Drug Snapshot Series, We Take a Closer Look at the Adoption Patterns and Future Prospects of Fezolinetant
Menopause symptoms have been historically overlooked and undermanaged in medical practice, an oversight that has led to insufficient treatment options and reflects a broader neglect of women's health issues. Of these symptoms, hot flashes are the most commonly reported. An estimated 75% to 80% of women in the U.S. report experiencing them around the time of their menopausal transition. Yet treatment options are limited, and hot flashes remain largely untreated even among women who describe them as “severe.”
A new drug is helping to change that. Veozah™ (fezolinetant), approved by the FDA in early 2023, represents a significant advancement in hot-flash treatment options. Veozah is a neurokinin 3 (NK3) receptor antagonist that interrupts the signaling pathway in the brain that is thought to contribute to vasomotor symptoms like hot flashes. Prior to its introduction, the most common treatment for menopause symptoms, particularly hot flashes, was hormone replacement therapy (HRT), and nonhormonal options were limited to antidepressants, blood pressure medications, supplements, and lifestyle changes.
Our new analysis explores the initial uptake of Veozah across the healthcare landscape and examined its potential for wider adoption. This analysis was produced using Komodo's MapLab™ solution, which rapidly generates high-value insights and leverages out-of-the-box healthcare-specific analytics on a unified platform. Several MapLab™ templates were used, including the Incidence and Prevalence template to visualize easily-digestible patient breakdowns, the Cohort Report template to identify patient groups by age, location, and payer channel, and the HCP Referral Network Analysis template to examine referring HCPs by specialty.
Here’s what we found:
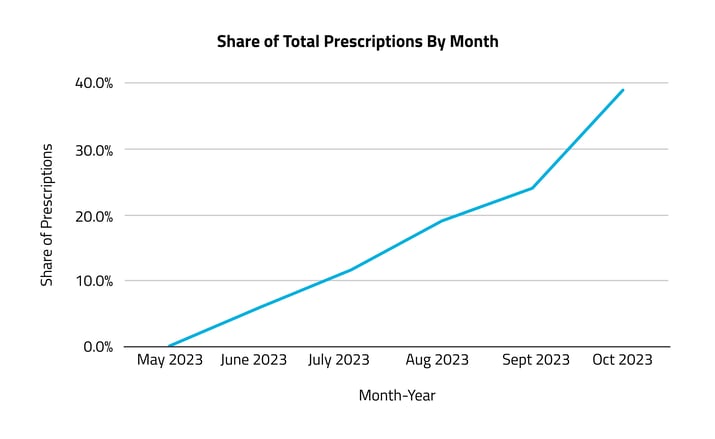
From its market debut in 2023, Veozah prescriptions experienced a steady rise, from just under 900 in June to more than 5,000 by October.
Patients most often received their prescriptions from an OB/gyns (34%), while other top-prescribing providers included nurse practitioners (22%), internal medicine physicians (17%), family medicine physicians (15%), and physician assistants (7%).
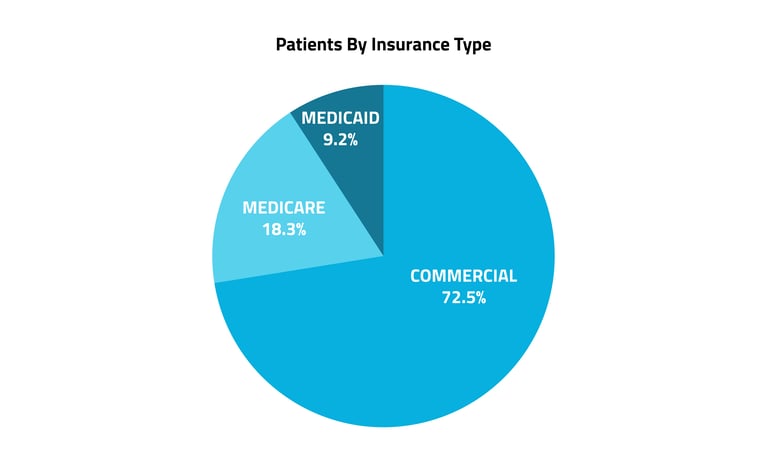
Insurance played a large role in determining which patients would be the first to receive this novel treatment. Commercial insurance accounted for 73% of Veozah recipients, followed by Medicare, 18%, and Medicaid, 9%. Geographically, Kentucky, Louisiana, Alaska, Delaware, and Alabama had the most patients with prescriptions per capita.
The strong rate of growth elucidated by this analysis indicates a positive reception of Veozah among healthcare providers. However, the potential for growth in market penetration among its target demographic is large. If we consider a rate of 20% for women who report severe hot flashes, that’s 1.32 million of the roughly 6.6 million women between the ages of 49 and 51 — a two-year period leading up to the average age of menopause. Given this conservative estimate, Veozah has a 0.43% market penetration as of October 2023, indicating a significant opportunity for increased adoption.
Continued monitoring of Veozah's adoption using advanced analytics tools is crucial to supporting Life Sciences organizations in transforming menopausal care. Komodo will continue providing insights that inform healthcare strategies to ensure that effective treatments reach the patients most in need.
Check out some of Komodo’s other rapidly generated Fast Facts featuring up-to-date insights on disease, treatment, and care based on real-world data from our full-stack healthcare analytics platform.
To see more articles like this, follow Komodo Health on X, LinkedIn, or YouTube, and visit Insights on our website.







