Race & Ethnicity in Clinical Trials: Breaking the Status Quo

Health equity has moved squarely to the center of our industry’s collective consciousness over the last few years as countless examples of racial, ethnic, and socioeconomic disparities in care have cultivated growing frustration and widespread mistrust of the U.S. healthcare system.
While the issue is fraught with challenges that include everything from access to care to institutional shortcomings in healthcare payment structures, there is one foundational fact that needs to be addressed before we can ever make substantive progress. People of color have been consistently underrepresented in clinical trials and have experienced worse outcomes and higher disease risk for decades. In fact, according to our research, 85% of oncology clinical trial participants over the last five years have been White.
That’s a big problem.
New Strategies to Close Gaps in Representation
Whether the phenomenon has been driven by a disproportionate reliance of clinical research teams on a handful of research hospitals located in major city centers, lack of awareness, lack of trust, outright systemic racism, or some combination of all of these, people of color in the U.S. continue to face an unequal burden of disease. We can take steps to remedy this imbalance. In addition to regulatory guidance by the FDA and advocacy support from clinical oversight organizations, technology solutions can help to turn this trend around. Data, analytics, and powerful software applications can optimize clinical trial design, spur recruitment, and improve outcomes for underrepresented patient populations.
At Komodo, we’ve enabled this data-driven approach to trial design and execution by expanding our Healthcare Map™ of de-identified real-world patient journeys to include hundreds of millions of self-identified race, ethnicity, and other demographic characteristics. Armed with this near-census level information, clinical teams are now able to better model the impact of their potential inclusion/exclusion criteria across different racial and ethnic groups, inform clinical endpoints by understanding the patient journeys of individuals across different racial and ethnic groups, identify diverse trial participants based on specific screening, diagnosis, referral, and treatment pathways, and improve site selection by identifying the providers who are actively treating patients with similar medical experiences from different racial and ethnic groups.
Unlocking the Truth With Enhanced Demographic Insights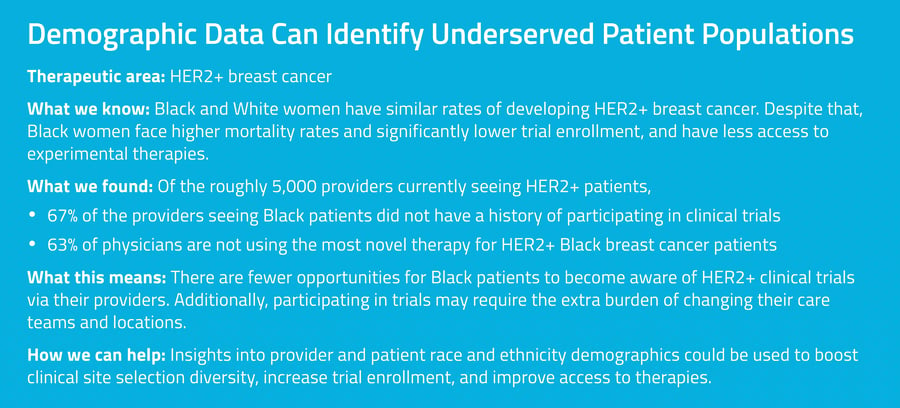
Improving Access and Optimizing Clinical Trial Sites With Demographic Data
We also looked at the racial distribution of breast, colon, pancreatic, and cervical cancer patients at the hospitals that are most likely to be chosen as clinical research sites. We started with this year’s U.S. News & World Report Best Hospitals for Cancer list. Currently, the hospitals on the list are collectively treating roughly 230,000 cancer patients. Of those, just 25% are patients of color. By considering other similar hospitals viable for clinical trials, not being anchored to traditional perceptions of influence and thought leadership, we are able to generate a list of hospitals that could be great targets for clinical trials and effectively bump the relative representation of patients of color by 45%.
Moving Past the Status Quo
By now, we can all agree that the old-fashioned approach to clinical trial design and recruitment is due for an overhaul. There was a time when building clinical strategies around a handful of known key opinion leaders at an elite subset of the total hospital universe was the gold standard. But advances in real-world patient data and analytics have opened up new opportunities to design clinical trials based on need – aligning protocol development, site selection, and patient recruitment based on precise patterns of patient behavior and medical history.
Only by refocusing clinical development to be based on patient experience rather than limited, legacy data, can we make it possible for clinical trials to become truly equitable.
About the Author: Kwame leads Komodo’s efforts to ensure customers maximize real-world evidence and advanced technology to optimize and accelerate clinical development. Prior to Komodo, he most recently spent 13 years at Genentech. Kwame has an MBA from the University of California, Berkeley, and a BS from Stanford University.
Connect with us to learn more about how Komodo's race & ethnicity demographic data can unlock novel insights and power Life Sciences strategies.
To see more articles like this, follow Komodo Health on Twitter, LinkedIn, or YouTube, and visit Insights on our website.





